Two iron porphyrin complexes with either mesityl (FeTMP) or thiophene (FeT3ThP) peripheral substituents were attached to basal pyrolytic graphite and Ag electrodes via different immobilization methods. By combining cyclic voltammetry and in-operando surface-enhanced Raman spectroscopy along with MD simulations and DFT calculations, their respective surface attachment, redox chemistry and activity toward electrocatalytic oxygen reduction was investigated. For both porphyrin complexes, it could be shown that catalytic activity is restricted to the first (few) molecular layer(s), although electrodes covered with thiophene-substituted complexes showed a better capability to consume the oxygen at a given overpotential even in thicker films. The spectroscopic data and simulations suggest that both porphyrin complexes attach to a Ag electrode surface in a way that maximum planarity and minimum distance between the catalytic iron site and the electrode is achieved. However, due to the distinctive design of the FeT3ThP complex, the thiophene rings are capable of occupying a conformation, via rotation around the bonding axis to the porphyrin, in which all four sulfur atoms can coordinate to the Ag surface. This effect creates a dense and planar surface coverage of the porphyrin on the electrode facilitating a fast (multi) electron transfer via several covalent Ag–S bonds. In contrast, bulky mesityl groups as peripheral substituents, which have been initially introduced to prevent aggregation and improve catalytic behavior in solution, exert a negative effect on the overall electrocatalytic performance in the immobilized state as a less dense coverage and less stable interactions with the surface are formed. Our results underline the importance of rationally designed heterogenized molecular catalysts to achieve optimal turnover, which not only strictly applies to the here discussed oxygen reduction reaction but eventually holds also true for other energy conversion reactions such as carbon dioxide reduction.
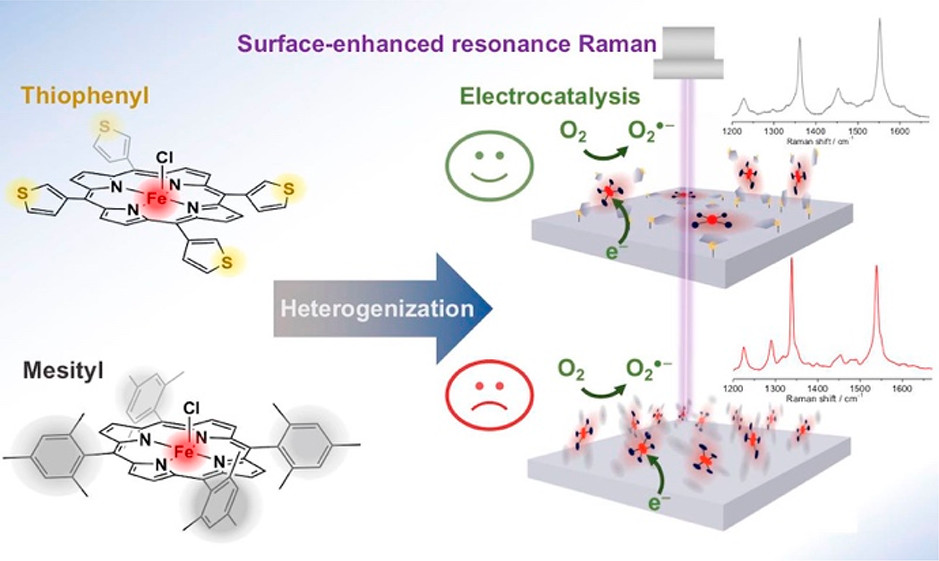
Two iron porphyrin complexes with either mesityl (FeTMP) or thiophene (FeT3ThP) peripheral substituents were attached to basal pyrolytic graphite and Ag electrodes via different immobilization methods. By combining cyclic voltammetry and in-operando surface-enhanced Raman spectroscopy along with MD simulations and DFT calculations, their respective surface attachment, redox chemistry and activity toward electrocatalytic oxygen reduction was investigated. For both porphyrin complexes, it could be shown that catalytic activity is restricted to the first (few) molecular layer(s), although electrodes covered with thiophene-substituted complexes showed a better capability to consume the oxygen at a given overpotential even in thicker films. The spectroscopic data and simulations suggest that both porphyrin complexes attach to a Ag electrode surface in a way that maximum planarity and minimum distance between the catalytic iron site and the electrode is achieved. However, due to the distinctive design of the FeT3ThP complex, the thiophene rings are capable of occupying a conformation, via rotation around the bonding axis to the porphyrin, in which all four sulfur atoms can coordinate to the Ag surface. This effect creates a dense and planar surface coverage of the porphyrin on the electrode facilitating a fast (multi) electron transfer via several covalent Ag–S bonds. In contrast, bulky mesityl groups as peripheral substituents, which have been initially introduced to prevent aggregation and improve catalytic behavior in solution, exert a negative effect on the overall electrocatalytic performance in the immobilized state as a less dense coverage and less stable interactions with the surface are formed. Our results underline the importance of rationally designed heterogenized molecular catalysts to achieve optimal turnover, which not only strictly applies to the here discussed oxygen reduction reaction but eventually holds also true for other energy conversion reactions such as carbon dioxide reduction.
