Diatoms are a significant group of algae displaying a sizeable morphological diversity, whose underlying structure arises from nanopatterned silica. Extensive experimental evidence suggests that a delicate interplay between various organic components and polysilicic acid plays a crucial role in biosilica mineralization. Thus, gaining insight into the properties of this organic–inorganic interface is of great interest in understanding the mechanisms controlling biosilica formation over different length scales. In this work, we use all-atom Molecular Dynamics simulations to investigate the aggregation behavior of polysilicic acid and silica nanoparticles in solution in the presence of protonated long-chain polyamines with a focus on the nature of the driving forces mediating the organic–inorganic aggregation process. Our results show that electrostatic forces between organic and inorganic species are the dominant interaction responsible for largely preserving the structural integrity of the organic–inorganic aggregates in solution. Thus, aggregates involving electrically neutral polysilicic acid are fully dissolved in an aqueous environment, since hydrogen bonding and van der Waals interactions turn out to be not strong enough to keep the aggregates together. Our main simulation results are in qualitative agreement with in vitro experiments, so that we expect they can contribute to shedding light on the initial stages of biosilica mineralization in diatoms.
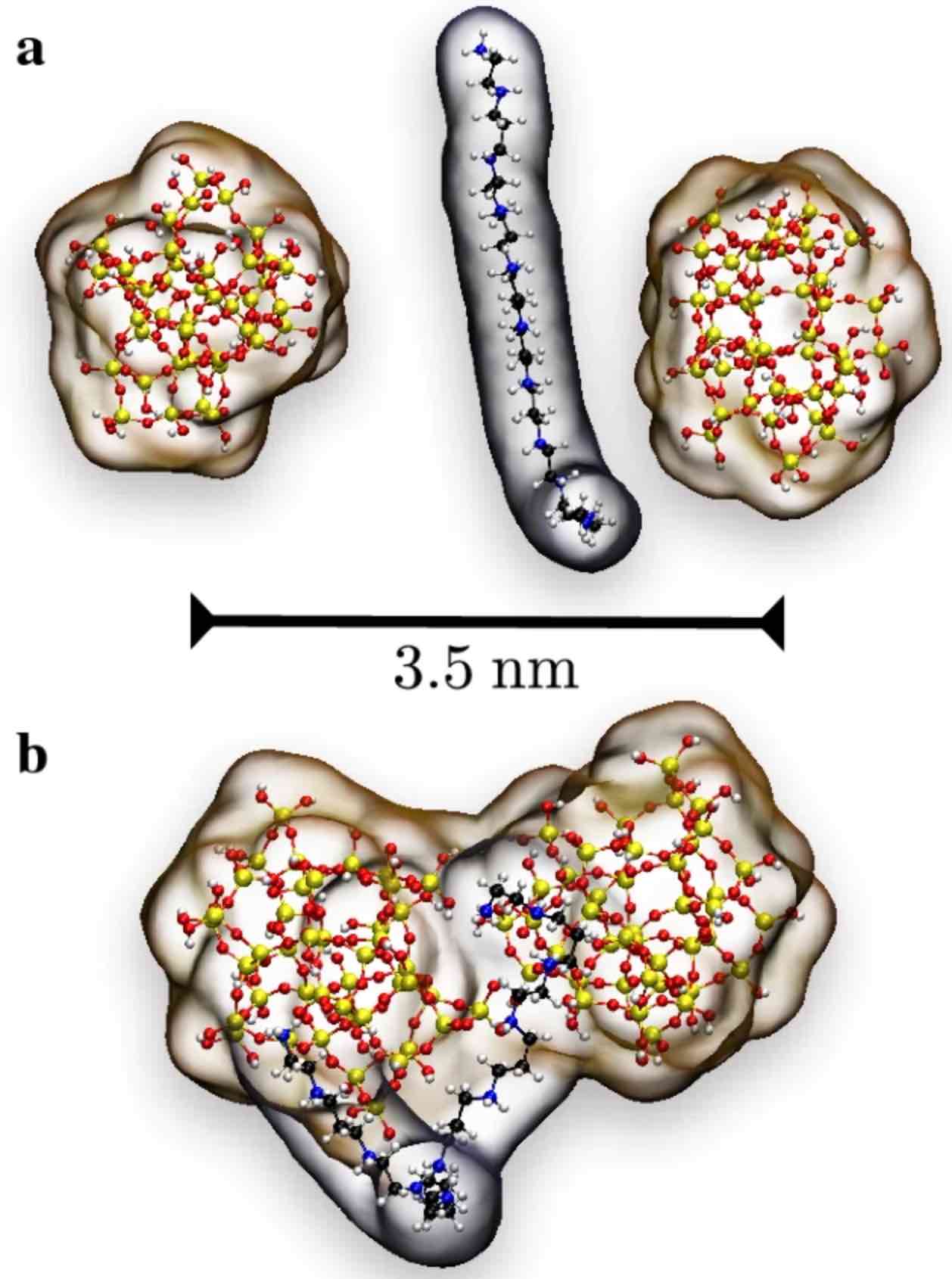
Diatoms are a significant group of algae displaying a sizeable morphological diversity, whose underlying structure arises from nanopatterned silica. Extensive experimental evidence suggests that a delicate interplay between various organic components and polysilicic acid plays a crucial role in biosilica mineralization. Thus, gaining insight into the properties of this organic–inorganic interface is of great interest in understanding the mechanisms controlling biosilica formation over different length scales. In this work, we use all-atom Molecular Dynamics simulations to investigate the aggregation behavior of polysilicic acid and silica nanoparticles in solution in the presence of protonated long-chain polyamines with a focus on the nature of the driving forces mediating the organic–inorganic aggregation process. Our results show that electrostatic forces between organic and inorganic species are the dominant interaction responsible for largely preserving the structural integrity of the organic–inorganic aggregates in solution. Thus, aggregates involving electrically neutral polysilicic acid are fully dissolved in an aqueous environment, since hydrogen bonding and van der Waals interactions turn out to be not strong enough to keep the aggregates together. Our main simulation results are in qualitative agreement with in vitro experiments, so that we expect they can contribute to shedding light on the initial stages of biosilica mineralization in diatoms.
