Understanding the diffusion of lithium ions in electrode materials for lithium ion batteries is of great importance for their knowledge-based optimization and development of novel materials and cell designs. The galvanostatic intermittent titration technique (GITT) is widely applied in battery research to study the diffusion of lithium in anode and cathode materials depending on the degree of lithiation. While transport properties of electrode materials at high and ambient temperatures are largely available, low temperature diffusion and rate coefficients are hardly reported in the literature and vary by orders of magnitude for identical active materials. Herein, we demonstrate and discuss several challenges and pitfalls in the application and evaluation of GITT measurements for determining the effective chemical lithium ion diffusion coefficient in lithium insertion electrodes, which become especially important at low temperature. This includes theoretical considerations and an experimental analysis of the promising cathode material LiNi0.5Co0.2Mn0.3O2 (NCM523) in the wide temperature range of -40 °C to 40 °C. We show how the choice of experimental conditions for the GITT measurements and of the subsequent mathematical evaluation significantly influence the derived diffusion coefficient. The results suggest that the large scattering of reported values of the diffusion coefficient could be caused by the use of different evaluation procedures. Simple calculation methods appear to be less suited the lower the temperature is. It is shown that the complementary use of GITT and EIS supplemented by detailed knowledge of the microstructure of the electrode significantly improves the accuracy of determining the diffusion coefficient.
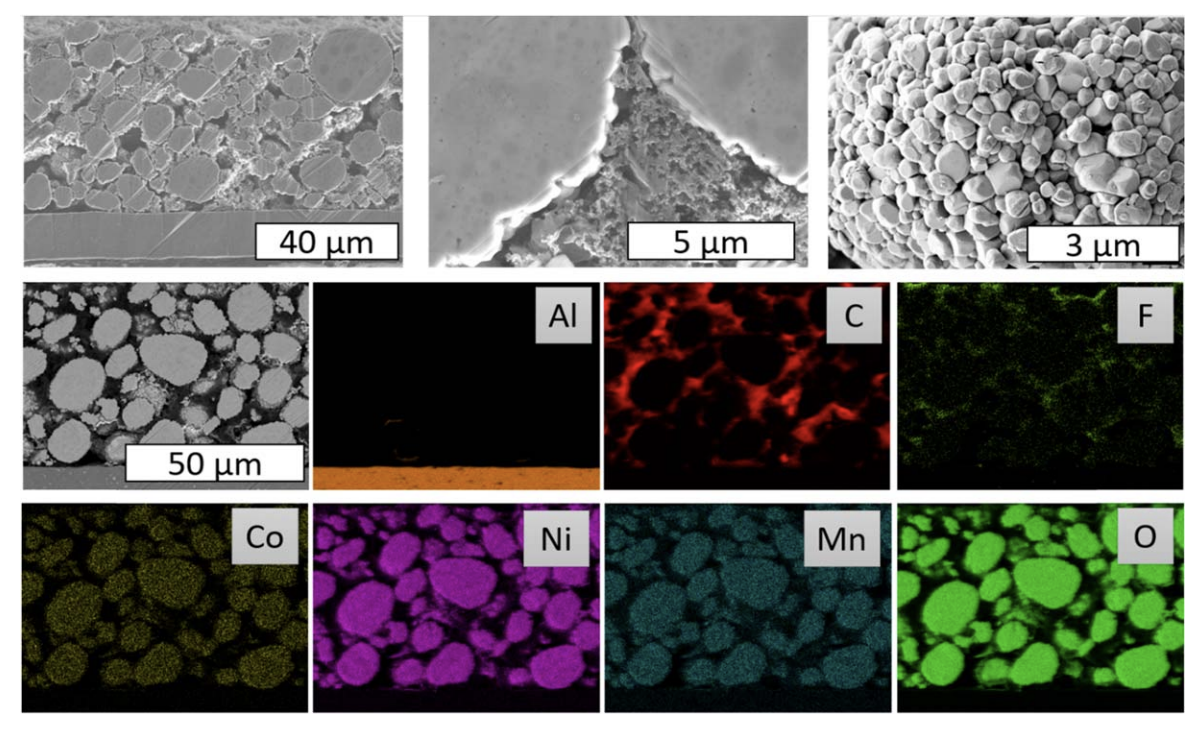
Understanding the diffusion of lithium ions in electrode materials for lithium ion batteries is of great importance for their knowledge-based optimization and development of novel materials and cell designs. The galvanostatic intermittent titration technique (GITT) is widely applied in battery research to study the diffusion of lithium in anode and cathode materials depending on the degree of lithiation. While transport properties of electrode materials at high and ambient temperatures are largely available, low temperature diffusion and rate coefficients are hardly reported in the literature and vary by orders of magnitude for identical active materials. Herein, we demonstrate and discuss several challenges and pitfalls in the application and evaluation of GITT measurements for determining the effective chemical lithium ion diffusion coefficient in lithium insertion electrodes, which become especially important at low temperature. This includes theoretical considerations and an experimental analysis of the promising cathode material LiNi0.5Co0.2Mn0.3O2 (NCM523) in the wide temperature range of -40 °C to 40 °C. We show how the choice of experimental conditions for the GITT measurements and of the subsequent mathematical evaluation significantly influence the derived diffusion coefficient. The results suggest that the large scattering of reported values of the diffusion coefficient could be caused by the use of different evaluation procedures. Simple calculation methods appear to be less suited the lower the temperature is. It is shown that the complementary use of GITT and EIS supplemented by detailed knowledge of the microstructure of the electrode significantly improves the accuracy of determining the diffusion coefficient.
