The efficient loading and sustained release of proteins from bioactive microspheres remain a significant challenge. In this study, we have developed bioactive microspheres which can be loaded with protein and then have a controlled rate of protein release into a surrounding medium. This was achieved by preparing a bioactive microsphere system with core-shell structure, combining a calcium silicate (CS) shell with an alginate (A) core by a one-step in situ method. The result was to improve the microspheres' protein adsorption and release, which yielded a highly bioactive material with potential uses in bone repair applications. The composition and the core-shell structure, as well as the formation mechanism of the obtained CS-A microspheres, were investigated by X-ray diffraction, optical microscopy, scanning electron microscopy, energy dispersive spectrometer dot and line-scanning analysis. The protein loading efficiency reached 75 per cent in CS-A microspheres with a core-shell structure by the in situ method. This is significantly higher than that of pure A or CS-A microspheres prepared by non-in situ method, which lack a core-shell structure. CS-A microspheres with a core-shell structure showed a significant decrease in the burst release of proteins, maintaining sustained release profile in phosphate-buffered saline (PBS) at both pH 7.4 and 4.3, compared with the controls. The protein release from CS-A microspheres is predominantly controlled by a Fickian diffusion mechanism. The CS-A microspheres with a core-shell structure were shown to have improved apatite-mineralization in simulated body fluids compared with the controls, most probably owing to the existence of bioactive CS shell on the surface of the microspheres. Our results indicate that the core-shell structure of CS-A microspheres play an important role in enhancing protein delivery and mineralization, which makes these composite materials promising candidates for application in bone tissue regeneration.
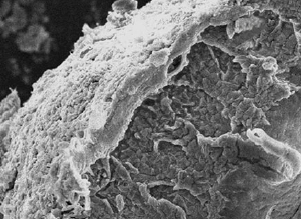
The efficient loading and sustained release of proteins from bioactive microspheres remain a significant challenge. In this study, we have developed bioactive microspheres which can be loaded with protein and then have a controlled rate of protein release into a surrounding medium. This was achieved by preparing a bioactive microsphere system with core-shell structure, combining a calcium silicate (CS) shell with an alginate (A) core by a one-step in situ method. The result was to improve the microspheres' protein adsorption and release, which yielded a highly bioactive material with potential uses in bone repair applications. The composition and the core-shell structure, as well as the formation mechanism of the obtained CS-A microspheres, were investigated by X-ray diffraction, optical microscopy, scanning electron microscopy, energy dispersive spectrometer dot and line-scanning analysis. The protein loading efficiency reached 75 per cent in CS-A microspheres with a core-shell structure by the in situ method. This is significantly higher than that of pure A or CS-A microspheres prepared by non-in situ method, which lack a core-shell structure. CS-A microspheres with a core-shell structure showed a significant decrease in the burst release of proteins, maintaining sustained release profile in phosphate-buffered saline (PBS) at both pH 7.4 and 4.3, compared with the controls. The protein release from CS-A microspheres is predominantly controlled by a Fickian diffusion mechanism. The CS-A microspheres with a core-shell structure were shown to have improved apatite-mineralization in simulated body fluids compared with the controls, most probably owing to the existence of bioactive CS shell on the surface of the microspheres. Our results indicate that the core-shell structure of CS-A microspheres play an important role in enhancing protein delivery and mineralization, which makes these composite materials promising candidates for application in bone tissue regeneration.
