We report on the coexistence of 2 different supramolecular polymorphic forms of pepsin-digested collagen type I fibrils reconstituted in vitro in the presence of heparin. Detailed structural analysis using transmission electron microscopy and scanning force microscopy shows a hierarchy involving 3 different structural levels and banding patterns in the system: asymmetric segment longspacing (SLS) fibrils and symmetric segments with an average periodicity (AP) of 250-260 nm, symmetric fibrous longspacing (FLS IV) nanofibrils with AP of 165 nm, and cofibrils exhibiting an asymmetric D-periodicity of 67 nm with a striking resemblance to the native collagen type I banding pattern. The intercalation of the high negatively charged heparin in the fibrils is suggested as being the main trigger for the hierarchical formation of the polymorphic structures. We propose a model explaining the unexpected presence of a symmetric and asymmetric form in the system and the principles governing the symmetric or asymmetric fate of the molecules.
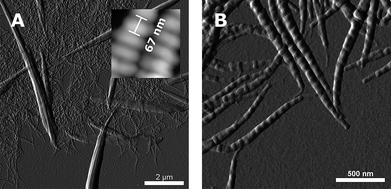
We report on the coexistence of 2 different supramolecular polymorphic forms of pepsin-digested collagen type I fibrils reconstituted in vitro in the presence of heparin. Detailed structural analysis using transmission electron microscopy and scanning force microscopy shows a hierarchy involving 3 different structural levels and banding patterns in the system: asymmetric segment longspacing (SLS) fibrils and symmetric segments with an average periodicity (AP) of 250-260 nm, symmetric fibrous longspacing (FLS IV) nanofibrils with AP of 165 nm, and cofibrils exhibiting an asymmetric D-periodicity of 67 nm with a striking resemblance to the native collagen type I banding pattern. The intercalation of the high negatively charged heparin in the fibrils is suggested as being the main trigger for the hierarchical formation of the polymorphic structures. We propose a model explaining the unexpected presence of a symmetric and asymmetric form in the system and the principles governing the symmetric or asymmetric fate of the molecules.
