In contrast with empty fullerenes, nitride clusterfullerenes usually exhibit irreversible reduction steps at moderate electrochemical scan rates. However, these reduction steps are chemically reversible, indicating that reversible follow-up reaction takes place. To explain this phenomenon, we analyze in this work if anion-radicals of nitride clusterfullerenes are more prone to dimerization than anion-radicals of empty fullerenes. Extensive DFT computations are performed to find the most stable dianionic dimeric structures of Sc3N@C68, Sc3N@C80, Sc3N@C80(CF3)2, [5,6] and [6,6] pyrrolidine adducts of Sc3N@C80 and Y3N@C80, a series of Y3N@C2n (2n = 78, 80, 84, 86, 88), as well as those of empty fullerenes C60, C70, and C84. Dimerization energies of the most stable isomers are computed in the gas phase, with the use of van der Waals corrections, and in solution. It is found that dianionic dimers of nonderivatized nitride clusterfullerenes are substantially more stable than those of empty fullerenes, which can be an explanation of the electrochemical irreversibility of the former.
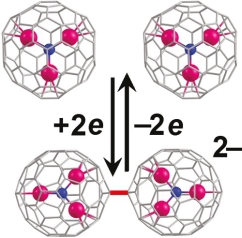
In contrast with empty fullerenes, nitride clusterfullerenes usually exhibit irreversible reduction steps at moderate electrochemical scan rates. However, these reduction steps are chemically reversible, indicating that reversible follow-up reaction takes place. To explain this phenomenon, we analyze in this work if anion-radicals of nitride clusterfullerenes are more prone to dimerization than anion-radicals of empty fullerenes. Extensive DFT computations are performed to find the most stable dianionic dimeric structures of Sc3N@C68, Sc3N@C80, Sc3N@C80(CF3)2, [5,6] and [6,6] pyrrolidine adducts of Sc3N@C80 and Y3N@C80, a series of Y3N@C2n (2n = 78, 80, 84, 86, 88), as well as those of empty fullerenes C60, C70, and C84. Dimerization energies of the most stable isomers are computed in the gas phase, with the use of van der Waals corrections, and in solution. It is found that dianionic dimers of nonderivatized nitride clusterfullerenes are substantially more stable than those of empty fullerenes, which can be an explanation of the electrochemical irreversibility of the former.
