On-surface synthesis provides a powerful method for the generation of long acene molecules, making possible the detailed investigation of the electronic properties of single higher acenes on a surface. By means of scanning tunneling microscopy and spectroscopy combined with theoretical considerations, we discuss the polyradical character of the ground state of higher acenes as a function of the number of linearly fused benzene rings. We present energy and spatial mapping of the tunneling resonances of hexacene, heptacene, and decacene, and discuss the role of molecular orbitals in the observed tunneling conductance maps. We show that the energy gap between the first electronic tunneling resonances below and above the Fermi energy stabilizes to a finite value, determined by a first diradical electronic perturbative contribution to the polyacene electronic ground state. Up to decacene, the main contributor to the ground state of acenes remains the lowest-energy closed-shell electronic configuration.
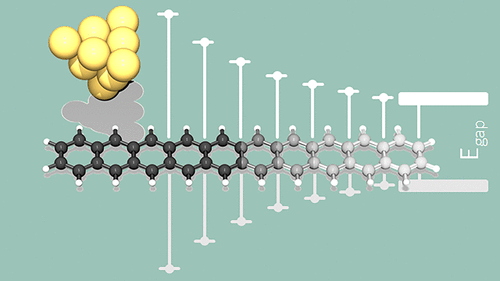
On-surface synthesis provides a powerful method for the generation of long acene molecules, making possible the detailed investigation of the electronic properties of single higher acenes on a surface. By means of scanning tunneling microscopy and spectroscopy combined with theoretical considerations, we discuss the polyradical character of the ground state of higher acenes as a function of the number of linearly fused benzene rings. We present energy and spatial mapping of the tunneling resonances of hexacene, heptacene, and decacene, and discuss the role of molecular orbitals in the observed tunneling conductance maps. We show that the energy gap between the first electronic tunneling resonances below and above the Fermi energy stabilizes to a finite value, determined by a first diradical electronic perturbative contribution to the polyacene electronic ground state. Up to decacene, the main contributor to the ground state of acenes remains the lowest-energy closed-shell electronic configuration.
