We investigated the thermally induced on-surface cyclization of 4,10-bis(2’-bromo-4’-methylphenyl)-1,3-dimethylpyrene to form the previously unknown, non-alternant polyaromatic hydrocarbon diindeno[1,2,3-cd:1´,2´,3´-mn]pyrene on Au(111) using scanning tunneling microscopy and spectroscopy. The observed unimolecular reaction involves thermally induced debromination followed by selective ring-closure to fuse the neighboring benzene moieties via a five-membered ring. The structure of the product has been verified experimentally as well as theoretically. Our results demonstrate that on-surface reactions give rise to unusual chemical reactivities and selectivities and provide access to new molecules.
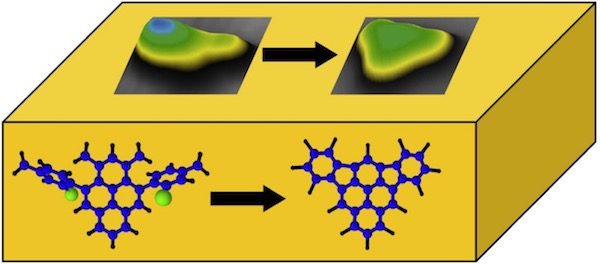
We investigated the thermally induced on-surface cyclization of 4,10-bis(2’-bromo-4’-methylphenyl)-1,3-dimethylpyrene to form the previously unknown, non-alternant polyaromatic hydrocarbon diindeno[1,2,3-cd:1´,2´,3´-mn]pyrene on Au(111) using scanning tunneling microscopy and spectroscopy. The observed unimolecular reaction involves thermally induced debromination followed by selective ring-closure to fuse the neighboring benzene moieties via a five-membered ring. The structure of the product has been verified experimentally as well as theoretically. Our results demonstrate that on-surface reactions give rise to unusual chemical reactivities and selectivities and provide access to new molecules.
