The SARS-CoV-2 pandemic has increased the demand for low-cost, portable, and rapid biosensors, driving huge research efforts toward new nanomaterial-based approaches with high sensitivity. Many of them employ antibodies as bioreceptors, which have a costly development process that requires animal facilities. Recently, sybodies emerged as a new alternative class of synthetic binders and receptors with high antigen binding efficiency, improved chemical stability, and lower production costs via animal-free methods. Their smaller size is an important asset to consider in combination with ultrasensitive field-effect transistors (FETs) as transducers, which respond more intensely when biorecognition occurs near their surface. This work demonstrates the immobilization of sybodies against the spike protein of the virus on silicon surfaces, which are often integral parts of the semiconducting channel of FETs. Immobilized sybodies maintain the capability to capture antigens, even at low concentrations in the femtomolar range, as observed by fluorescence microscopy. Finally, the first proof of concept of sybody-modified FET sensing is provided using a nanoscopic silicon net as the sensitive area where the sybodies are immobilized. The future development of further sybodies against other biomarkers and their generalization in biosensors could be critical to decrease the cost of biodetection platforms in future pandemics.
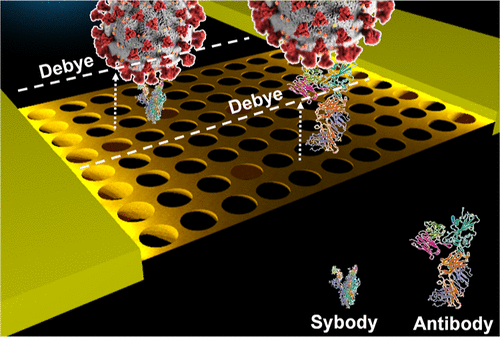
The SARS-CoV-2 pandemic has increased the demand for low-cost, portable, and rapid biosensors, driving huge research efforts toward new nanomaterial-based approaches with high sensitivity. Many of them employ antibodies as bioreceptors, which have a costly development process that requires animal facilities. Recently, sybodies emerged as a new alternative class of synthetic binders and receptors with high antigen binding efficiency, improved chemical stability, and lower production costs via animal-free methods. Their smaller size is an important asset to consider in combination with ultrasensitive field-effect transistors (FETs) as transducers, which respond more intensely when biorecognition occurs near their surface. This work demonstrates the immobilization of sybodies against the spike protein of the virus on silicon surfaces, which are often integral parts of the semiconducting channel of FETs. Immobilized sybodies maintain the capability to capture antigens, even at low concentrations in the femtomolar range, as observed by fluorescence microscopy. Finally, the first proof of concept of sybody-modified FET sensing is provided using a nanoscopic silicon net as the sensitive area where the sybodies are immobilized. The future development of further sybodies against other biomarkers and their generalization in biosensors could be critical to decrease the cost of biodetection platforms in future pandemics.
