Despite favorable absorption characteristics, borondipyrromethenes (BODIPYs) often lack thermal stability preventing their application in vacuum-processed organic solar cells. In this paper, the replacement of the BF2 unit by borafluorene as a new functionalization strategy for this molecule class is explored. This approach is applied to a set of prototype molecules and demonstrates improved thermal stability, strong absorption in the red and near-infrared region of the sun spectrum, as well as excellent solar cell performance. Synthesis is realized from free ligands via complexation with 9-chloro-9-borafluorene giving high yields up to 81%. Planar heterojunction cells of these complexes exhibit high fill factors of more than 70%. Bulk heterojunction solar cells with C-60 are optimized yielding power conversion efficiencies up to 4.5%, rendering the investigated prototype compounds highly competitive among other NIR-absorbing small-molecule donor materials. Comprehensive experimental material characterization and solar cell analysis are carried out, and the results are discussed together with simulations of molecular properties. Based on this analysis, additional performance improvements are proposed by engineering the intramolecular steric interactions towards further red-shifted absorption.
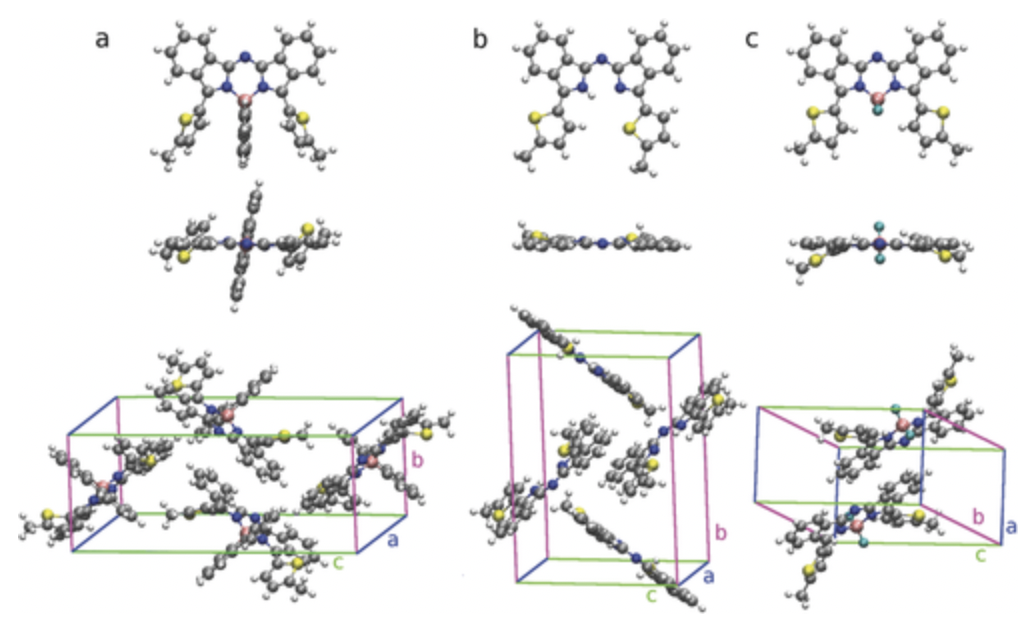
Despite favorable absorption characteristics, borondipyrromethenes (BODIPYs) often lack thermal stability preventing their application in vacuum-processed organic solar cells. In this paper, the replacement of the BF2 unit by borafluorene as a new functionalization strategy for this molecule class is explored. This approach is applied to a set of prototype molecules and demonstrates improved thermal stability, strong absorption in the red and near-infrared region of the sun spectrum, as well as excellent solar cell performance. Synthesis is realized from free ligands via complexation with 9-chloro-9-borafluorene giving high yields up to 81%. Planar heterojunction cells of these complexes exhibit high fill factors of more than 70%. Bulk heterojunction solar cells with C-60 are optimized yielding power conversion efficiencies up to 4.5%, rendering the investigated prototype compounds highly competitive among other NIR-absorbing small-molecule donor materials. Comprehensive experimental material characterization and solar cell analysis are carried out, and the results are discussed together with simulations of molecular properties. Based on this analysis, additional performance improvements are proposed by engineering the intramolecular steric interactions towards further red-shifted absorption.
